Can Suspensions of Particles (Including Gel Particles) Show Better Placement Properties than Gelants When Used as Blocking Agents?
(No, except under rare circumstances.)
Placement
Several researchers proposed the use of particles as blocking agents (see Refs. 104-111 in Ref. 13). With particles, two different approaches can be taken to control placement. The first approach is commonly used in matrix acidizing.65 If they are large enough relative to pore throats, particles can form a filter cake on the rock surfaces. Since the largest volume of the injected suspension enters the most-permeable zone, the largest filter cake forms on that zone, and that filter cake can restrict flow to the greatest extent in the most-permeable zone. However, at best this method will equalize injection rates in the different zones.65 If too much of the suspension is injected, flow will be restricted in all zones. Also, any beneficial flow diversion occurs at the wellbore. If flow is reversed (e.g., return of an oil well to production), the filter cake can be removed, and the effect of the diverting agent will be reversed. Finally, if this diversion method is combined with another blocking agent, such as a gelant, an undesirable placement results—the gelant is diverted into rather than away from the less-permeable zones.
The second placement approach using particles (Fig. 23) relies on the relation between the particle size and the pore sizes of the zones of interest. In concept, a suspension of particles could penetrate readily into a high-permeability zone, while the particles are removed by filtration on the rock faces of less-permeable zones. If the fluid contains a gelant or other blocking agent, that blocking agent could be selectively placed in the high-permeability zone with minimum penetration into less-permeable zones.
For this second concept to work, several requirements must be fulfilled. First, the particles must be small enough to penetrate freely into the most-permeable zones. Second, the particles must be large enough to form an external filter cake on the rock surface of the less-permeable zones. Third, the particle size distribution must be sufficiently narrow. We developed a theoretical model to study the feasibility of using particles to prevent gelant penetration into low-permeability zones during the placement process.13 Our analysis indicated that to achieve selective placement using particles with a normal size distribution, there is a maximum standard deviation of particle sizes that should not be exceeded for a given permeability contrast. For example, consider two zones with permeabilities of 10,000 md and 100 md, respectively. Assume a best-case scenario where all particles less than 33.3 Ám in diameter will flow freely through the 10,000-md rock and where all particles greater than 3.33 Ám in diameter will form an external filter cake on the 100-md rock. Therefore, if monodisperse particles were available, a selective placement of a blocking agent could be achieved using any particles that were smaller than 33.3 Ám and larger than 3.33 Ám
In reality, particles in a given suspension have a distribution of sizes. We have shown13 that for a given standard deviation of a normal particle-size distribution, the maximum selectivity for placement of a blocking agent is achieved by choosing the average of the critical particle sizes of the high- and low-permeability zones as the mean particle size [in this example, (33.3+3.33)/2 = 18.3 Ám]. If particles with a mean size of 18 Ám are used in our example, the standard deviation of the size distribution must be smaller than 9 Ám to achieve better selectivity than a water-like gelant without particles.13 To achieve the same selectivity as particles with a monodisperse size of 18 Ám, the standard deviation must be smaller than 4 Ám. The maximum allowable standard deviation for selective placement decreases with decreasing permeability contrast.13
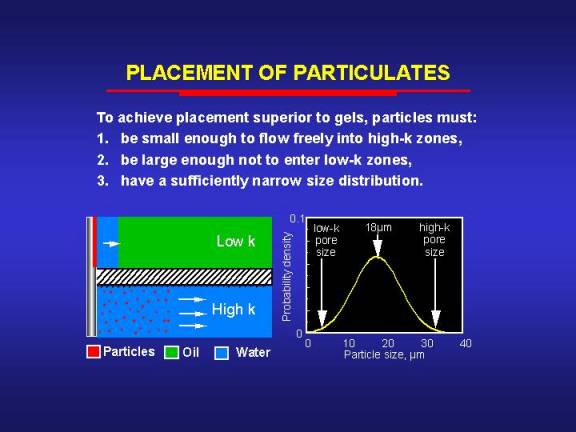
The above analysis is actually optimistic since it assumes that the rock has a single pore size. Because porous media contain a range of pore sizes, the particles used must have a narrower size distribution than was indicated above to achieve selectivity during placement.13 The utility of particles in controlling placement of blocking agents may also be limited by other factors that were not considered in our simple model. In particular, the ability of particles to penetrate into a given porous medium also depends on the influence of fluid velocity, particle concentration, and the surface chemistries of the particles and porous media.66-68
For intermediate-sized particles (those small enough to flow readily into the high-permeability zone but large enough not to enter the low-permeability zone), the relative distance of penetration, in concept, could be zero. On the surface, this behavior suggests a tremendous placement advantage over gelants. However, if the particles flow freely through the high-permeability rock, they may not provide a significant permeability reduction. Therefore, the particles by themselves are not expected to be an effective blocking agent in the high-permeability zones.
The above shortcoming could be remedied by incorporating a gelant or similar blocking agent with the suspended particles.13 Intermediate-sized particles suspended in a gelant could readily enter the high-permeability zone. However, the particles would form a filter-cake on the surface of the low-permeability zone—thus, minimizing gelant penetration. Of course, some gelant will inevitably enter the low-permeability zone during placement. Even so, the potential exists to achieve a substantially better placement than that possible with a gelant alone.
Proper particle sizing is extremely important when combining particles with gelants. If particles are small enough to penetrate readily into all zones, gelant placement will be no better than that for a low-viscosity gelant without particles. If the particles are too large to penetrate into any zone, external filter cakes will form on all zones, and an excessive amount of gelant could enter the low-permeability zone (as expected from fluid diversion concepts in matrix acidizing65). In fact, the gelant could penetrate almost as far in the low-permeability zone as in the high-permeability zone.13
Permeability Reduction
The degree of permeability reduction caused by particles can be separated into two components: (1) that associated with an external filter cake formed at the surface of a given zone and (2) that associated with an "internal filter cake" formed from particles trapped inside the porous medium. Because the external filter cake can be removed or circumvented by mechanical means (e.g., jet washing, backflow, or perforation), we are concerned primarily with the permeability reduction associated with the internal filter cake. For particles trapped inside a porous medium, the degree of permeability reduction qualitatively follows the same trend as that for weak gels. In particular, formation damage factors or residual resistance factors tend to increase with increasing ratio of particle size to pore size.69-71 (This behavior has been reported when the ratio of particle size to pore size ranges from 1/14 to 1/3.71) This parallel in behavior between particles and weak gels is not surprising since weak gels usually consist of a suspension of gel aggregates, which are a specific form of particulate. In concept, the potential improvements in placement that were discussed above with regard to particles could also be achieved using suspensions of gel aggregates. Of course, the limitations also apply. An extensive analysis by Midha et al.57 indicates that suspensions of gel aggregates will be no more selective than gelants.
Hypothetically, particles could reduce the flow capacity of water zones to a greater extent than oil zones. Small particles could be injected that are soluble in oil but not soluble in water.72,73 These particles must be sized so that they enter the porous rock and become trapped by deep-bed filtration. Upon returning the well to production, the particles could significantly reduce the permeability of watered-out zones. In contrast, in zones with high fractional oil flows, the particles may quickly dissolve—thus restoring a high oil permeability.