Mechanistic Implications
This section summarizes our current views of the mechanism for disproportionate permeability reduction for relatively “strong” (pore-filling) gels such as Cr(III)-acetate-HPAM.
Water Injected First after Gel Placement
Immediately after placement and gelation, the water-based gel occupies all of the aqueous pore space. Residual oil may be trapped in pore centers in water-wet rock such as Berea (see the schematic in Figure 24). In oil-wet porous media (e.g., porous polyethylene), low mobility “residual oil” may coat pore walls. If water or brine is injected after gel placement, it must flow through the gel itself (Figure 24). Since the inherent permeability to water is in the microdarcy range for flow through the gel, a very large permeability reduction is observed.20 For rock with an initial permeability (before gel) around one darcy, the water residual resistance factor can be greater than 10,000. Thus, the gel can greatly reduce flow from gel-invaded water zones.
Oil Injected First after Gel Placement
Oil is typically the first fluid that contacts the gel-treated region when a well is returned to production. We found that oil reduces the pore volume occupied by gel. This volume reduction created pathways for oil flow, thus restoring an important level of permeability to oil. The schematic in Figure 25 illustrates this process.
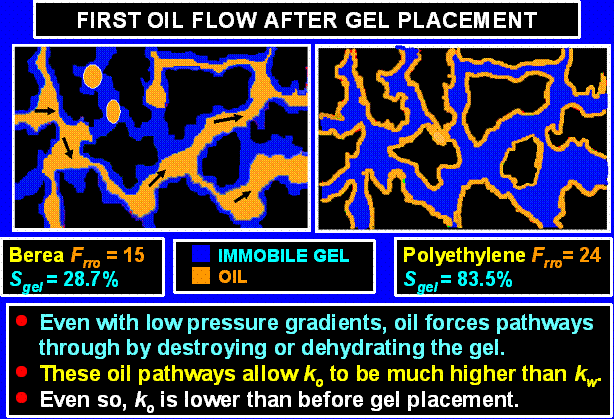
When oil is injected, how does a reduction in gel volume occur? Several possibilities come to mind, including oil (a) ripping through the gel, (b) concentrating or dehydrating the gel, (c) mobilizing the gel, or (d) chemically destroying the gel. As our oil (hexadecane) was not reactive with any of the gel, brine, or rock components, possibility d), chemical destruction of the gel, does not seem likely. As we have never observed gel production from our cores, we view possibility c) as unlikely. However, it is possible that gel particles too small for us to detect were displaced from the core.
That leaves two mechanisms for active consideration. In one mechanism, oil ripped pathways through the gel.1,7,21,22 In the second mechanism,23-25 gel dehydrated.
Our recent analysis supports the dehydration mechanism over the ripping or gel mobilization mechanisms. In particular, the apparent reduction in gel saturation during oil injection was insensitive to pore size in Berea (Figure 14) and was greatest in small pores in porous polyethylene (Figure 20). If ripping or gel mobilization were the dominant mechanisms, losses in gel volume should have been greatest in the largest pores. To explain, if gel failure (i.e., ripping or gel extrusion) occurred at a gel-rock interface or within the gel, force balance analysis suggests that the pressure gradient for gel failure should be inversely proportional to the pore radius.22,26,27 Thus, for a given applied pressure gradient, gel failure should occur dominantly in larger pores. Since this did not occur, our results argue against the ripping or gel mobilization mechanisms.
In contrast, the observed XMT results could be consistent with the dehydration mechanism. With a fixed pressure gradient applied through the porous medium, gel in all pores could be “squeezed” or dehydrated to the same extent, regardless of pore size.
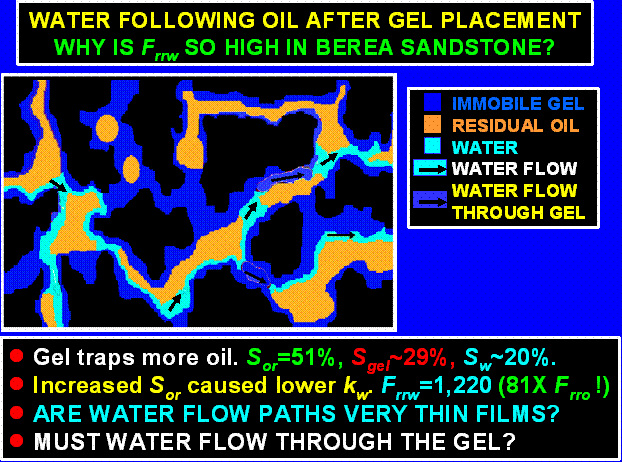
Water Following Oil Flow after Gel Placement in Berea.
If an oil zone that was treated with gel eventually waters out, our results indicate that the water residual resistance factors will be substantially greater than the residual resistance factors observed during the previous oil flow. What mechanism can explain this behavior? In water-wet Berea sandstone, our results indicate that a significantly higher level of residual oil is trapped during water flow (Figure 18 and Table 2). Even without the presence of gel, higher oil saturations necessarily lead to lower permeabilities to water. With higher oil saturations, water pathways would be more constricted. Given the large ratio of Frrw/Frro (81) in Berea, the primary pathways for water flow could conceivably be either thin water films and/or through the gel itself (see the schematic in Figure 26).
When water was reinjected to establish Sor after gel placement, gels conceivably could rehydrate and swell to some extent. Did this occur? Table 2 indicates that the water saturation (or the combined water plus gel saturation) in Berea changed from 63.7% immediately after gel placement, to 28.7% at Swr after gel to 49% at Sor after gel. If the decrease in Sw from 63.7% to 28.7% during oil injection was due to dehydration, the gel would have been concentrated by an average factor of 2.2. If the increase in Sw from 28.7% to 49% during water injection was entirely due to rehydration, the gel would have swelled by a factor of 1.7.
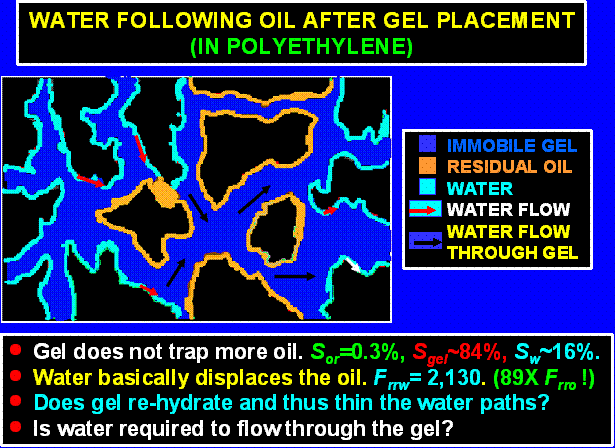
Water Following Oil Flow after Gel Placement in Polyethylene.
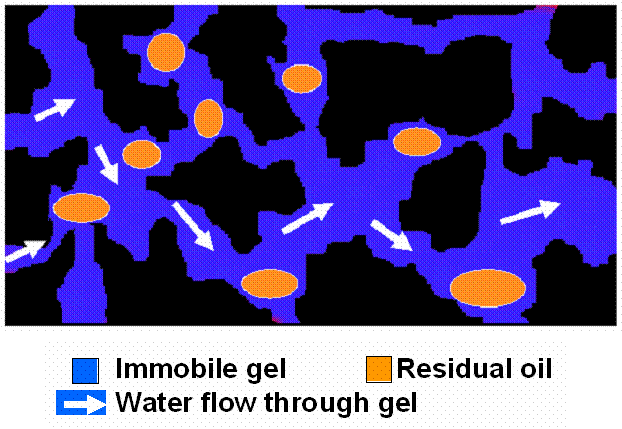
In contrast to the behavior in water-wet Berea, gel does not appear to trap high levels of residual oil during water flow following oil flow after gel placement (Table 2 and Figure 21). Nevertheless, our results in polyethylene indicated that the water residual resistance factors were still substantially greater than the residual resistance factors observed during the previous oil flow (Frrw/Frro =89). Similar to the case for Berea, the primary pathways for water flow could be either thin water films and/or through the gel itself (schematic in Figure 27).
Table 2 indicates that the water saturation (or the combined water plus gel saturation) in polyethylene changed from 99.8% immediately after gel placement, to 83.5% at Swr after gel to 99.7% at Sor after gel. If the decrease in Sw from 99.8% to 83.5% during oil injection was entirely due to dehydration, the gel would have been concentrated by a factor of 1.2—much less than observed in Berea. If the increase in Swfrom 83.5% to 99.7% during water injection was entirely due to rehydration, the gel would have swelled almost entirely back to its original size. Additional evidence of rehydration comes from the Frro and Frrw values for gel in the polyethylene core (Frro =24, Frrw =2,130). Since our earlier discussion suggested that oil and water may largely follow the same path, the high Frrwvalue could be explained by gel rehydration partially closing the path.