Gelant Injection: 20-cp Gelant Mobilized Oil in Both Porous Media
During gelant placement in Berea, the oil saturation in the imaged region increased, surprisingly, from 18.4% to 36.3%. (In other words, water saturation decreased from 81.6% to 63.7% in Figure 12.) To understand this result, note that the image volume was located in the center of the core and was small compared to the total pore volume of the core. Oil from upstream of the image volume was mobilized by flow of the 20-cp gelant, and that oil coincidentally lodged in the image volume. The overall oil saturation in the core did not change during gelant placement. (No oil was observed in the core effluent during gelant injection.) Within the imaged region, medium to large pores (10-4 to 10-2 mm3) were most likely to gain in oil saturation (Figure 12). The pressure gradient during gelant injection was always less than that during the previous brine or oil flows. This constraint was intentionally part of our experimental design to minimize oil mobilization. Since oil was mobilized, factors other than high pressure gradients were responsible for this mobilization.
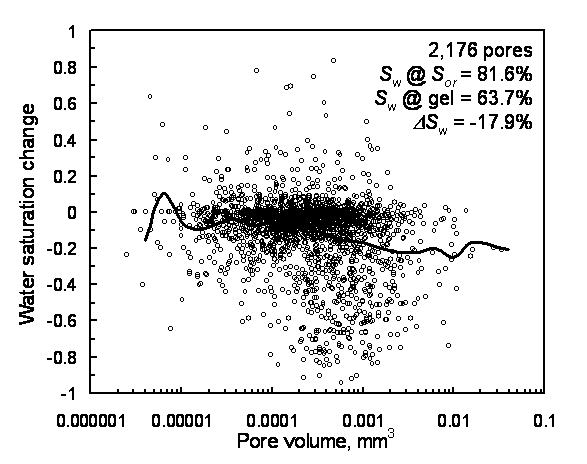
During gelant placement in polyethylene, the gelant or aqueous saturation increased from 83% to 99.8% (Table 2). Most oil that was trapped in small pores was displaced, so that most pores ended with high gelant saturations (compare Figures 7 and 13); only a few small pores retained large oil saturations (Figure 13). As in Berea, the pressure gradient during gelant injection was always less than that during the previous brine or oil flows. Again, no oil was observed in the core effluent during gelant injection.

Why was oil mobilized from the small pores during gelant injection but not during the previous water injection—especially since the pressure gradient during water injection (35 psi/ft) was higher than during gelant injection (23 psi/ft)? Could polymer or Cr(III) adsorption have altered the wettability (oil-wet to water-wet) of the polyethylene? This suggestion seems unlikely considering the hydrophobic nature of the surface and the hydrophilic nature of the polymer and crosslinker. Wang18 suggested that viscoelastic forces associated with flow of polymer solutions may re-distribute forces on a microscopic scale so that oil may be mobilized. This explanation may also help explain oil mobilization during our Berea experiments.
When gelant was placed, it effectively displaced all brine so that gel formed in all aqueous pore spaces. By itself, XMT cannot distinguish between water and gel. However, this observation has been confirmed many times in previous work by noting that the Cr(III)-acetate-HPAM gel reduced permeability to water (for both Berea and polyethylene) to levels associated with the permeability of the gel itself to water (i.e., final permeability in the microdarcy range).19,20